Abstract
Introduction: The approval of extended half-life recombinant factor IX (rFIX) replacement products has expanded the range of therapeutic options available for the treatment of hemophilia B. Compared with standard half-life FIX, these products allow for extended dosing intervals while maintaining appropriate bleed control. One such extended half-life product is rIX-FP (IDELVION; CSL Behring), a recombinant fusion protein linking rFIX with recombinant albumin, offering the possibility of dosing up to every 21 days in adults. The ATHN 2: Factor Switching Study provides information on patient outcomes following a switch from a previous FIX product to rIX-FP.
Methods: ATHN 2 was a factor-switching study sponsored by the American Thrombosis and Hemostasis Network (ATHN) conducted in participants across the US hemophilia treatment center (HTC) network. This was a multi-center, longitudinal, observational study with two arms: a prospective arm following participants for up to one year after switching factor replacement product, and a retrospective arm including participants who have switched products within the 50 weeks prior to enrollment with retrospective and/or prospective assessment for up to one year. Male and female children and adults (≥2 years) with FIX clotting activity ≤5% of normal who had previously been treated with plasma-derived or recombinant FIX for at least 50 exposure days were eligible for inclusion. Treatment was administered at the discretion of the participant's hemophilia caregiver. Data was collected at study/clinic visits, or via a telephone interview conducted every three months. Baseline demographic data was collected for all participants. The prescribed dosing frequency for each participant was collected before and after the switch to rIX-FP, including dosing frequency taken from the first and last treatment records taken during the study following the switch. Participants were also asked to rank their satisfaction with rIX-FP upon the completion or early termination of the study.
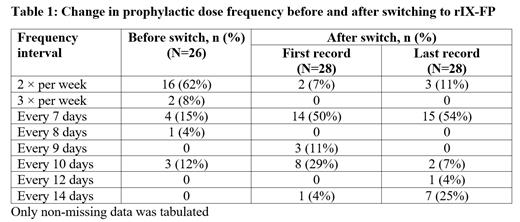
Results: A total of 41 participants were included in this analysis; 27 in the prospective arm and 14 in the retrospective arm. The mean (SD) age across all participants was 22.5 (17.1) years, ranging from 2 to 71 years. The median age was 18 years old and most participants had severe hemophilia B (FIX activity <1%; n=26, 63%). Prior to the switch to rIX-FP, 76% (n=31) of participants were receiving prophylaxis and 24% (n=10) received episodic treatment. The majority of participants (62%) receiving prophylaxis were treated twice a week (Table 1). Following the treatment switch, 93% of participants were initially assigned to a once-weekly or less frequent dosing regimen. This proportion remained stable over the course of the study, with 89% of participants on once-weekly or less frequent prophylaxis by the time of the last record taken. Among 23 participants with complete data on their prophylaxis dose interval before and after treatment switch, 70% of the participants were able to extend their dose frequency and maintain the extended dose frequency through the study. Thirty-seven participants responded to the satisfaction survey, with the majority (n=33, 89%) being somewhat or very satisfied with rIX-FP treatment.
Conclusions: Switching to rIX-FP allowed participants to extend their prophylaxis dosing interval and a majority of participants were able to maintain the extended dose interval through the study period. In addition, a majority of participants were satisfied with rIX-FP treatment, altogether suggesting that extended half-life factor replacement with rIX-FP offers a valuable treatment option for hemophilia B.
Journeycake: HEMA Biologics: Honoraria; LFB: Honoraria. Chrisentery-Singleton: Biomarin: Speakers Bureau; Kedrion: Consultancy; Takeda: Consultancy, Speakers Bureau; Spark: Consultancy, Research Funding; Sanofi: Consultancy; Pfizer: Consultancy, Research Funding, Speakers Bureau; Octapharma: Consultancy; Novo Nordisk: Consultancy, Speakers Bureau; Hema Biologics: Consultancy; Grifols: Consultancy; Genentech: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau. Desai: CSL Behring: Current Employment. von Drygalski: Pfizer: Research Funding; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hematherix, Inc: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Super FVa; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biomarin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; uniQure: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Patel: CSL Behring: Current Employment. Raffini: CSL Behring: Consultancy; Genentech: Consultancy; HEMA Biologics: Consultancy; Bayer: Consultancy; XaTek: Consultancy. Recht: Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; uniQure: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Pfizer: Consultancy; Octapharma: Consultancy; Novo Nordisk: Consultancy; Kedrion: Consultancy; Hema Biologics: Consultancy; Genentech: Consultancy; CSL Behring: Consultancy; American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment; Catalyst Biosciences: Consultancy. Sidinio: Biomarin: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy; Novo Nordisk: Consultancy; Bayer: Consultancy; Guardian Therapeutics: Consultancy; Octapharma: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Catalyst: Consultancy. Wang: Bayer: Consultancy; Biomarin: Consultancy; CSL Behring: Consultancy; Genentech: Consultancy; Hema Biologics: Consultancy; Novo Nordisk: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; uniQure: Consultancy. Zhang: CSL Behring: Current Employment, Current holder of individual stocks in a privately-held company. Neufeld: Pfizer: Consultancy; Chiesi: Consultancy; Bayer: Consultancy; Genentech: Consultancy; Octapharma: Consultancy, Research Funding; Acceleron Pharma: Consultancy; Baxter: Consultancy; Shire: Consultancy; Takeda: Consultancy; CSL: Consultancy; Biogen: Consultancy; Novo Nordisk: Consultancy; Bristol Myers-Squibb: Consultancy; ATHN: Research Funding; Celgene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal